Citotoxicity
Introduction
The increasing demand for environmentally friendly and sustainable agricultural practices has intensified interest in natural compounds with biopesticidal properties. Piperamides, naturally occurring alkaloids found in black pepper (Piper nigrum), have emerged as promising candidates due to their demonstrated insecticidal, antifungal, and antimicrobial activities. These properties make piperamides attractive for use as biopesticides in integrated pest management (IPM) strategies, which aim to reduce reliance on synthetic chemicals and minimize environmental impact.
To meet the growing need for such natural bioactive compounds, biotechnological production of piperamides using microbial systems has become a focal point of research. Saccharomyces cerevisiae, a well-characterized model organism for metabolic engineering, is an ideal host for the biosynthesis of piperamides. In this engineered pathway, ferulic acid serves as a critical intermediate metabolite. Although ferulic acid has its own bioactive properties, its primary role in this context is to serve as a precursor in the piperamide biosynthetic route. However, one of the key challenges in scaling up piperamide production in microbial systems is understanding the cytotoxic effects of both piperamides and ferulic acid on S. cerevisiae cells.
High concentrations of these compounds could potentially reduce cell viability, limiting the efficiency of the production process in bioreactors. Therefore, it is crucial to determine the concentration thresholds at which piperamides and ferulic acid begin to exert toxic effects on the host cells.
In this study, we conducted cytotoxicity assays using automated cell counting and flow cytometry to assess the impact of various concentrations of piperamides and ferulic acid on S. cerevisiae. The goal is to optimize the conditions for large-scale biopesticide production, ensuring that piperamide yields are maximized without compromising cell health. The insights gained from this research will contribute to the development of scalable, microbial-based production systems for piperamides, facilitating their use as sustainable biopesticides in agricultural applications.
Methods
Cytotoxicity assay - Automated Cell Counter
Materials
- S. cerevisiae
- Yeast Extract Peptone Dextrose (YPD) liquid medium
- Eppendorf tubes 1 mL
- Trypan Blue
- Eppendorf tube 0.2 mL
- Slides for Automated Cell Counter
Procedure
Growing S. cerevisiae in YPD liquid medium. Adjusted 1x106 cells/mL in every tube, adjusting to 1 mL of culture media to each tube. We cultivated 9 tubes in total.
Treatments
Table 1: Treatments description
| Treatment | Description | Concentration in culture medium | Solution applied (50μL) |
|---|---|---|---|
| NC_1 | Negative control 1 | NA | H2O |
| NC_2 | Negative control 2 | 20% | Etanol 100% |
| PC | Positive control | 6% | Hydrogen peroxide 30% |
| PS-1 | Piperamides | 0.02% | Piperamides solution 0.1% |
| PS-2 | Piperamides | 0.2% | Piperamides solution 1% |
| PS-3 | Piperamides | 2% | Piperamides solution 10% |
| FAS-1 | Ferulic acid | 0.004% | Ferulic acid solution at 0.02% |
| FAS-2 | Ferulic acid | 0.04% | Ferulic acid solution at 0.2% |
| FAS-3 | Ferulic acid | 0.4% | Ferulic acid solution at 2% |
We added the respective treatment (Table 1) to the respective tube and incubated the tubes at 28 degrees C for 45 minutes.
After the incubation took place, we took 10 microliters of the cell culture solution and added 10 microliters of Trypan Blue, mixing and adding that volume on a cell counter slide. We measured viability on every tube and registered the results.
Cytotoxicity assay - Flow Cytometry
Introduction
Here we describe the protocols we used to assess the piperamides and ferulic acid cytotoxic effects on S. cerevisiae cells using Flow Cytometry.
Materials
- S. cerevisiae culture
- 10 flow cytometry tubes
- Zombie NIR
- Paraformaldehyde
- PBS
- Culture media
Procedure
Growing S. cerevisiae culture: adjusted 1x106 cells/mL in every tube, adjusting to 1 mL of culture media in each tube. We cultivated 9 tubes in total.
Treatments
Table 2: Treatments description
| Treatment | Description | Concentration in culture medium | Solution applied (250μL) |
|---|---|---|---|
| H2O | Negative control 1 | NA | H2O |
| ET | Negative control 2 | 20% | Etanol 100% |
| PE | Positive control | 6% | Hydrogen peroxide 30% |
| P1 | Piperamides | 0.6% | Piperamides solution 3% |
| P2 | Piperamides | 1.2% | Piperamides solution 6% |
| P3 | Piperamides | 1.8% | Piperamides solution 9% |
| F1 | Ferulic acid | 0.000004% | Ferulic acid solution at 0.00002% |
| F2 | Ferulic acid | 0.00004% | Ferulic acid solution at 0.0002% |
| F3 | Ferulic acid | 0.0004% | Ferulic acid solution at 0.002% |
We added the respective treatment (Table 2) to the respective tube and incubated the tubes at 28°C for 45 minutes.
ZombieNIRTM Dye
After the incubation took place, we added 1 mL of culture media to every tube and washed the cells, resuspending 500 µL of PBS. The lights were turned off, and 300 µL of ZombieNIRTM dye (diluted 1:2000) was added to each tube.
The tubes were incubated for 15 minutes in darkness at room temperature. After incubation, we added 1 mL of culture media and washed the cells, resuspending them in 500 µL of paraformaldehyde. The cells were kept at 4°C, wrapped with aluminum foil.
FACS Discover S8 Becton Dickinson cytometry lecture
The day after, the cells from each tube were analyzed on the FACS Discover S8 Becton Dickinson Flow Cytometer.
Results
Cytotoxicity activity of piperamides and ferulic acid - Automated Cell Counter
Cytotoxicity was assessed through the measurement of viability of the cell cultures after these were exposed to the treatments (different concentrations of piperamides and ferulic acid) and incubated for 45 minutes. The viability was measured using an automated cell counter and trypan blue.
We did this exploratory assay in order to find the concentrations that were cytotoxic to the cells.
We started assessing the concentrations of ferulic acid beginning around the limit of solubility (0.4% m/v) of this compound in water.1
For the case of piperamides, we just started to assess with the concentration that can be found in the black pepper plant.2
After the first assay was conducted, we decided the concentrations to test in a cytotoxic assay made through flow cytometry.
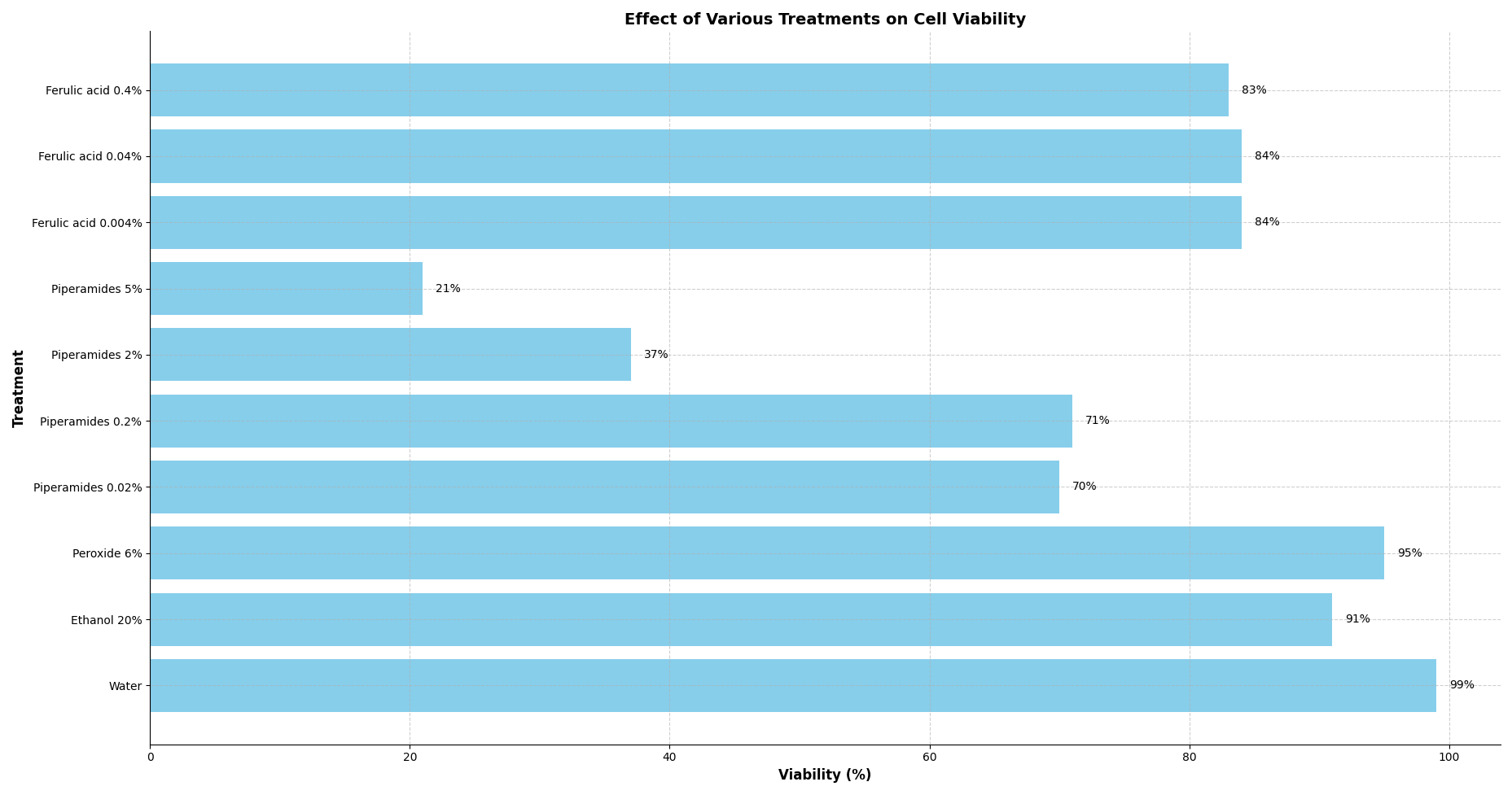
The first assay measurements of viability are on Figure 1. Here we can conclude that piperamides do have a cytotoxic effect on yeast cells, and also that the concentration that has a cytotoxic effect is between 0.2% and 2%.
The concentrations of ferulic acid that we tried show almost the same cell viability, so this range of concentrations maintains the cell viability around 84%.
These results guide us to try the concentrations in the following experiment.
Cytotoxicity activity of piperamides and ferulic acid - Flow Cytometry Assay
The cells were assessed using the FACS Discover S8 Becton Dickinson Flow Cytometer.
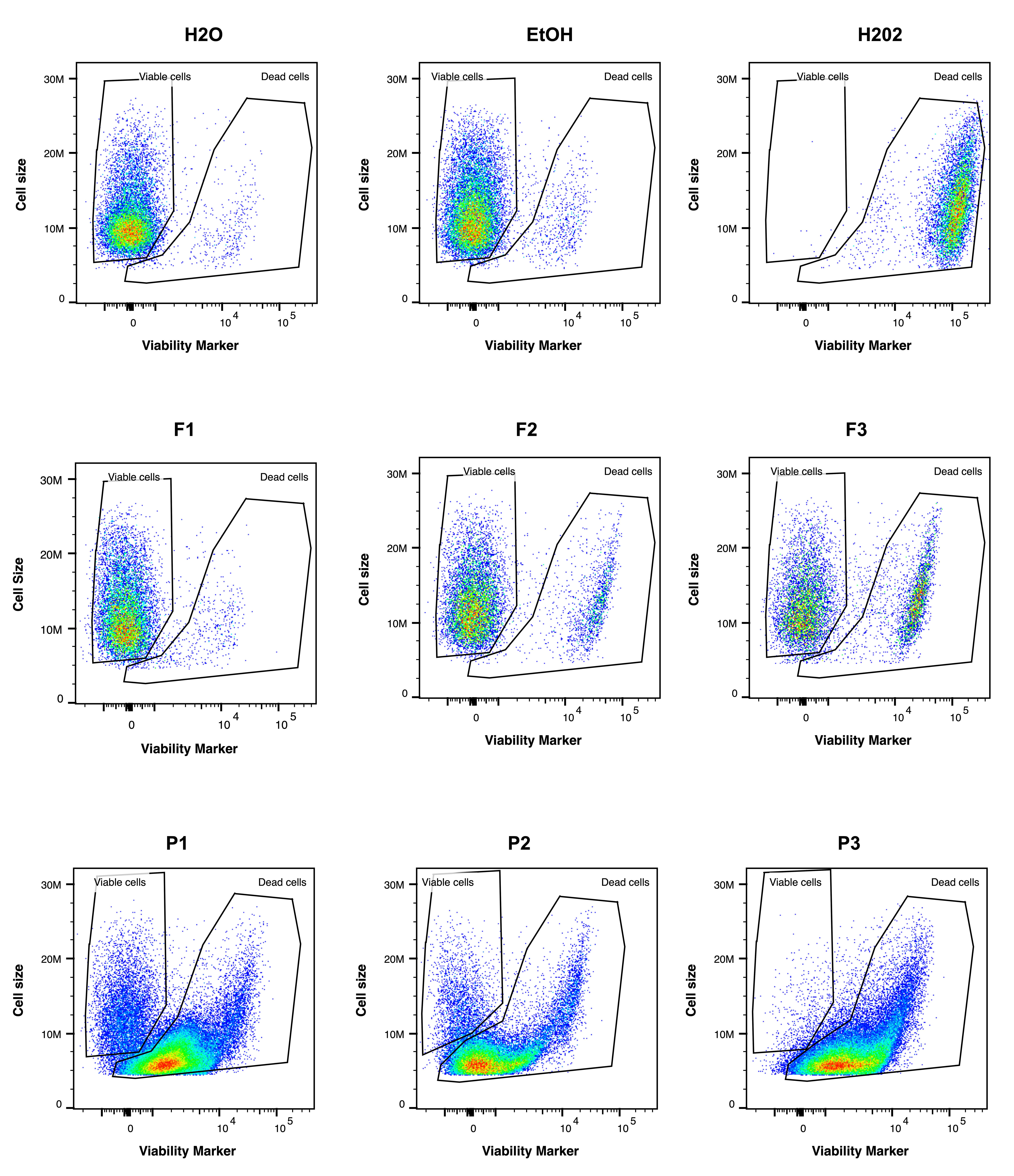
Table 1. Viability and Dead Cells percentage related to every treatment applied.
| Sample | Concentration | Viability (%) | Dead Cells (%) |
|---|---|---|---|
| H2O | NA | 95.1 | 2.79 |
| H2O2 | 6% | 0.13 | 99.2 |
| EtOH | 20% | 94.0 | 4.04 |
| F1 | 0.1% | 93.5 | 4.10 |
| F2 | 1% | 84.0 | 14.1 |
| F3 | 4% | 64.0 | 32.4 |
| P1 | 0.6% | 9.91 | 86.2 |
| P2 | 1.2% | 6.95 | 90.5 |
| P3 | 1.8% | 1.06 | 96.7 |
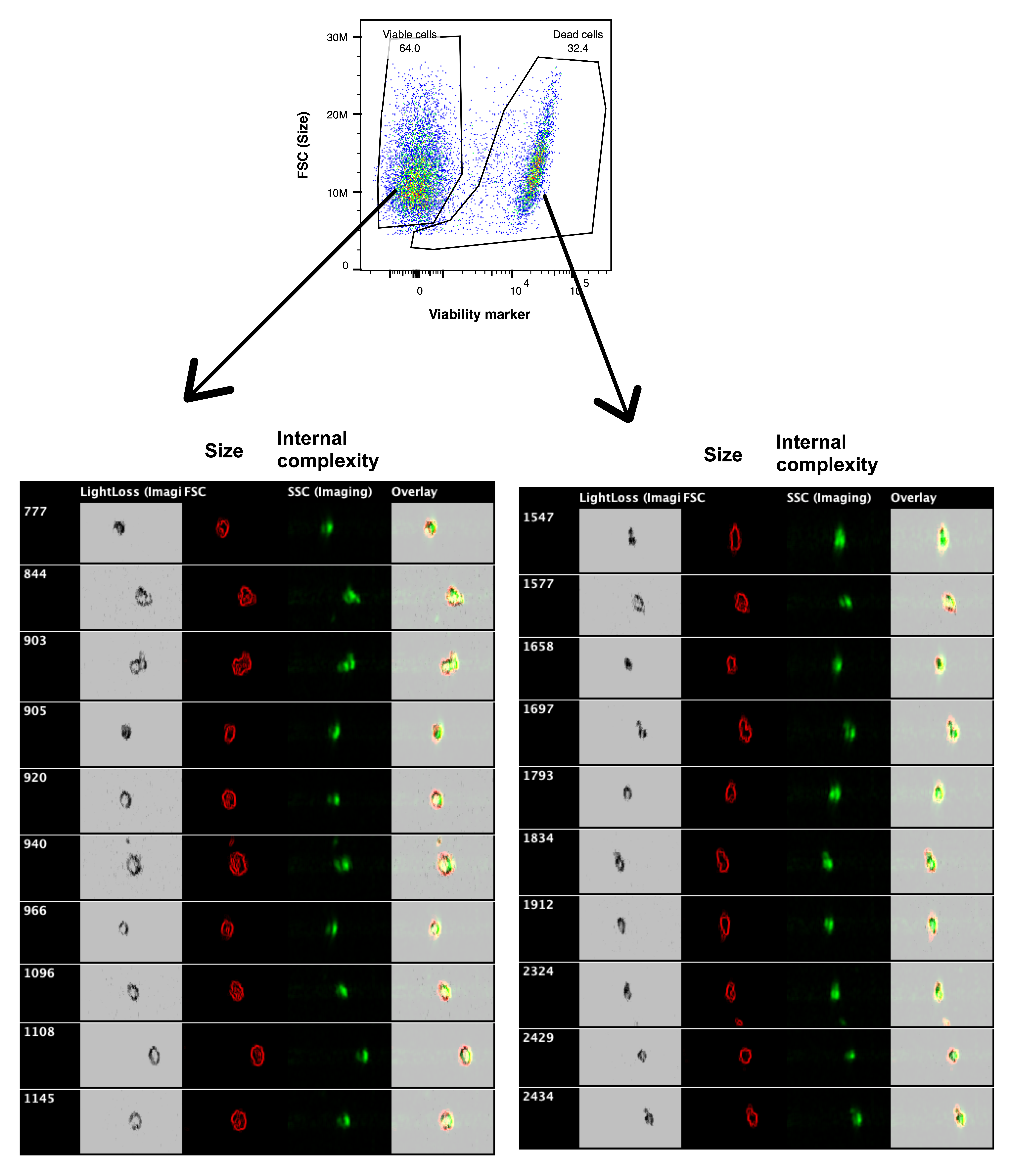
In Figure 3 we can see the differences between the live and dead cells. The live cells fraction shows bigger and more regular shape than the dead cells fraction.
Based on these exploratory assays, we can conclude that both ferulic acid and piperamides exhibit a cytotoxic effect on yeast cells. However, further assays are needed to more precisely identify the inflection points of their cytotoxicity.
Discussion
In this study, we investigated the cytotoxic effects of piperamides and ferulic acid on Saccharomyces cerevisiae to assess their suitability in metabolic pathway design for biosynthesis applications. Using both automated cell counting with trypan blue exclusion and flow cytometry with Zombie NIR™ viability dye, we quantified cell viability across a range of concentrations for both compounds.
Our initial assays using the automated cell counter indicated that piperamides exhibit significant cytotoxicity at higher concentrations. Specifically, at concentrations of 2% and 5%, cell viability decreased markedly to 37% and 21%, respectively (Figure 1). In contrast, ferulic acid did not show significant cytotoxic effects across the tested concentrations up to 0.4%, maintaining cell viability around 84%. These results suggest that piperamides are inherently more toxic to yeast cells than ferulic acid at equivalent concentrations.
Flow cytometry assays corroborated these findings and provided more detailed insights. Piperamides at concentrations of 0.6%, 1.2%, and 1.8% resulted in cell viabilities of 9.91%, 6.95%, and 1.06%, respectively (Table 1). The steep decline in viability at concentrations above 0.6% indicates a pronounced cytotoxic effect, emphasizing the need to carefully regulate intracellular levels of piperamides in engineered yeast strains. Ferulic acid demonstrated a dose-dependent but less severe cytotoxicity. Even at the highest concentration tested (0.0004%), cell viability remained at 64%, suggesting that yeast cells can tolerate higher concentrations of ferulic acid compared to piperamides.
An unexpected observation was the high viability in the positive control (6% hydrogen peroxide) during the automated cell counter assay, which contradicted established knowledge of hydrogen peroxide's oxidative cytotoxicity. This anomaly could be due to experimental error, such as incorrect reagent concentration, or it might reflect an adaptive oxidative stress response in yeast cells. However, in the flow cytometry assay, the hydrogen peroxide control resulted in expected cytotoxicity, with cell viability dropping to 0.13% and dead cells comprising 99.2% of the population. This discrepancy highlights the importance of assay validation and suggests that flow cytometry provides a more accurate assessment of cell viability in this context.
The morphological differences between live and dead cells observed via flow cytometry further confirmed the cytotoxic effects of the compounds tested. Live cells displayed larger sizes and greater internal complexity, while dead cells were smaller with irregular shapes (Figure 3). These structural changes are consistent with cellular responses to toxic stress and support the quantitative viability data obtained.
Our findings have significant implications for the metabolic engineering of S. cerevisiae for the biosynthesis of piperamides and ferulic acid. The cytotoxicity of piperamides at relatively low concentrations suggests that overproduction or accumulation within the cells could compromise cell viability and, consequently, the efficiency of bioproduction processes. Strategies such as dynamic regulation of pathway expression, efflux pumps to export piperamides, or compartmentalization might be necessary to mitigate cytotoxic effects. In contrast, the higher tolerance of yeast cells to ferulic acid indicates that its biosynthesis might be more feasible without extensive cellular modifications.
Limitations of this study include the need for more precise determination of the cytotoxic concentration thresholds for both compounds. For piperamides, further assays with concentrations between 0.2% and 0.6% could help identify the inflection point where cytotoxicity becomes significant. Similarly, for ferulic acid, testing concentrations between 0.01% and 1% would refine our understanding of its impact on cell viability. Additionally, investigating the mechanisms underlying the cytotoxic effects—such as membrane disruption, reactive oxygen species generation, or metabolic interference—could provide deeper insights and inform engineering strategies.
Conclusion
In conclusion, while ferulic acid appears to be a suitable intermediate for yeast-based biosynthesis with minimal cytotoxicity, piperamides pose a challenge due to their pronounced toxic effects at higher concentrations. Future work should focus on optimizing yeast strains and metabolic pathways to balance efficient production with cell health, ensuring sustainable biotechnological applications of these compounds.
References
1. Mota, F. et al. (2008). Aqueous Solubility of Some Natural Phenolic Compounds. Industrial and Engineering Chemistry Research, 47 (15), pp.5182-5189. 10.1021/ie074520. hal-01637105
2. Ikan, R. (1991). Natural Products: A Laboratory Guide. Academic Press.