Overview
Introduction
There is a compound derived from black pepper (Piper nigrum L.) called piperine. Results have been obtained that indicate that at concentrations of 10 g/L and 20 g/L piperine has a pesticide capacity that inhibits the hatching and development of fall armyworm larvae, mainly of the most recently laid eggs, causing egg mortality of up to 88%. Recent studies on the feasibility of plant extracts as pesticides against larvae of lepidoptera that infect common agricultural crops have suggested that in addition to piperine compounds such as β and α-asarone can have a pesticidal effect on Spodoptera litura larvae, a species related to the fall armyworm. It was found that the concentrations adequate to ensure the death of 50% of the larvae are 81.5, 6.24 and 2.22 micrograms per larva for peperin, β and α-asarone respectively. A synergistic effect was also shown in the biopesticide effect between extracts of P. retrofractum and A. calamus, whose main components are piperine for P. retrofractum (15.6%) and β and α-asarone, in a ratio of 4.38:1 for A. calamus.
Piperine is synthesized from two precursors: piperoyl-CoA and piperidine, these are condensed by the enzyme piperine synthase to form piperine. Both precursors are synthesized from amino acids, piperoyl-CoA has phenylalanine as precursor and piperidine comes from lysine. The piperine biosynthetic pathway in the black pepper is not completely determined.
The main goal of the project is to develop a biopesticide based on the piperamides present in the black pepper. It has been reported that a protein highly expressed in black pepper fruits similar to piperine synthase, called piperamide synthase, has a promiscuous substrate specificity and can accept different CoA donors to condense with piperidine and form a piperamide. One of the compounds that can be produced is a piperamide with a similar structure to piperine but with two fewer carbons; this is the result of using 3,4-Methylenedioxy cinnamoyl CoA as a CoA donor instead of piperoyl CoA. In order to see if this new piperamide could be a better option for a safe bio-pesticide active compound, we computed physicochemical descriptors and ADME parameters of both molecules using the SwissADME tool. One of the most important parameters calculated is skin permeability, which was computed using the QSPR model implemented by Abraham et al. (1995). The piperamide resulted with a lower skin permeability log kp (-5.88 cm/s) than piperine (-5.58 cm/s), making the selected piperamide a better option since it will permeate less the skin of the farmers who use a piperamide-based bio-pesticide. It also presents a better synthetic accessibility (2.59) compared to piperine (2.92).
However, as the metabolic pathway for the production of piperine and other piperamides remains incompletely elucidated, we devised a strategy to propose candidate enzymes capable of executing the enzymatic steps required in the metabolic pathway for the synthesis of the selected piperamide, as illustrated in the following image.
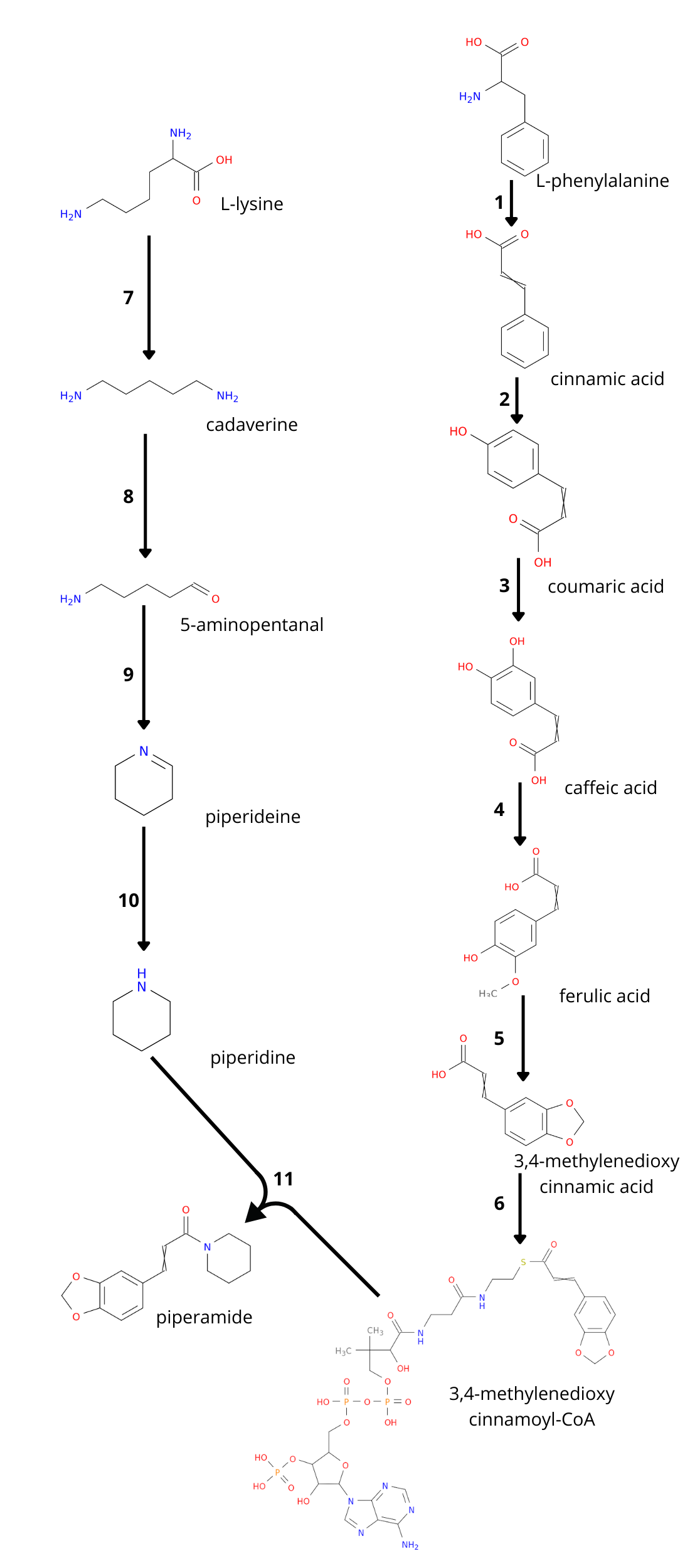
Figure X: piperamide
Methodology
In the next diagram we show a general pipeline of the work made in silico. The main goal of this roadmap was to propose candidate enzymes for the steps in the pathway. In order to achieve this goal we first performed the genome annotation of the genome of Piper nigrum, this was made since the available genome does not present annotation for the predicted genes. We then performed a Differential Expression Analysis using RNAseq data from different plant tissues to get the genes overexpressed in the mature fruit and the correct annotation, a similar strategy has been used by Schnabel, 2021, to discover another enzyme in the route, the piperine synthase. These genes were then used for molecular docking to see their binding affinity to the substrates corresponding to the enzyme number; this was a validation step for the proposed enzymes.
With the proposed enzymes a search for alternative candidate enzymes was performed, which started with a homology search in public databases, this was performed with the aim of getting a pool of sequences from which to get the alternative candidates. These sequences were analyzed with clustering methods to have selected enzymes to represent groups of enzymes within the universe of enzymes found by homology. The final list of alternative candidate enzymes was selected using both the homology search and the cluster analysis. The resulting set of selected alternatives was analyzed with molecular docking with the corresponding substrate and later Molecular Dynamics Simulations (MDS) was performed to compare the binding energy and stability of the substrate among the different candidate enzymes.
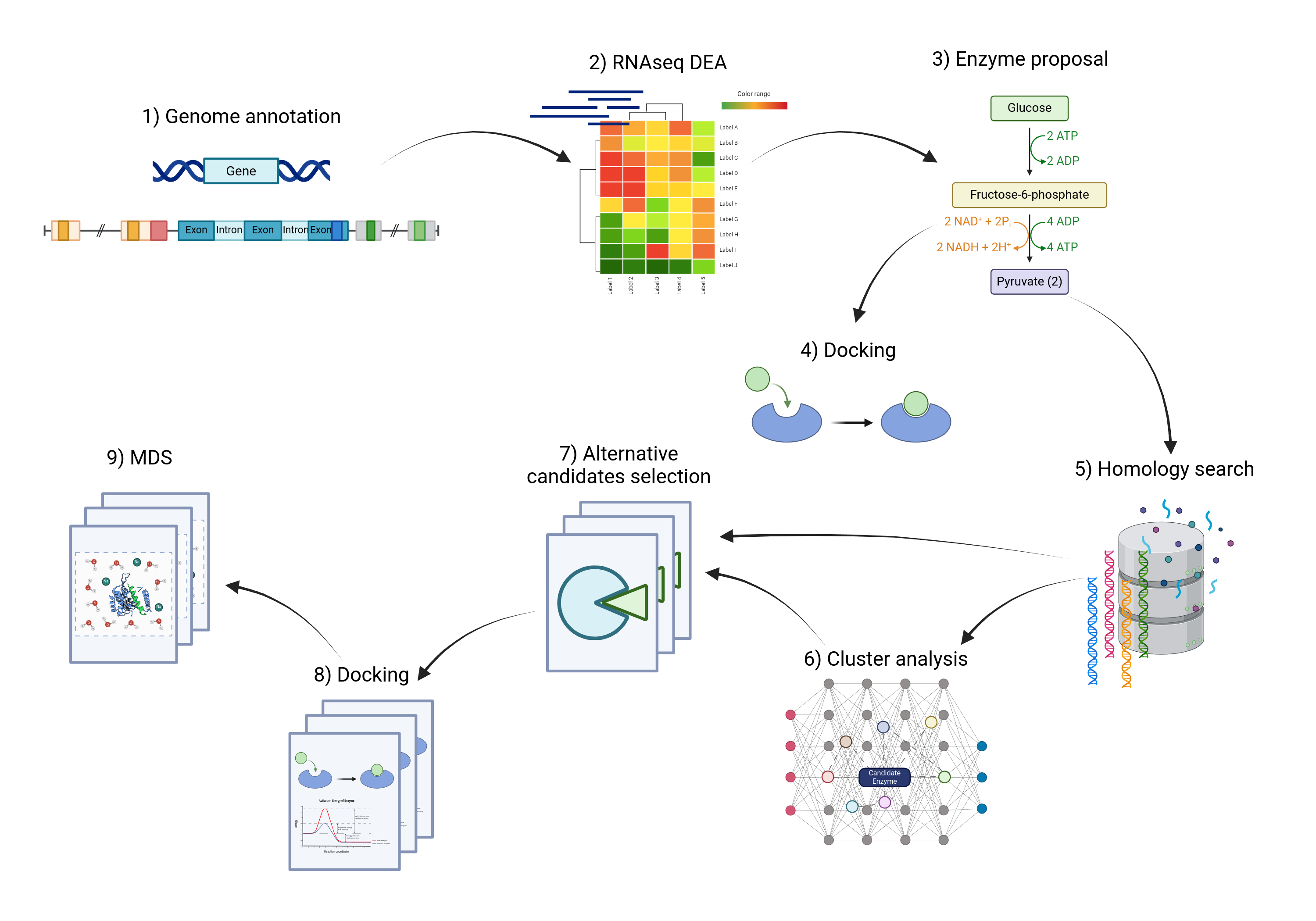
Figure X: General pipeline of the in silico work.